|
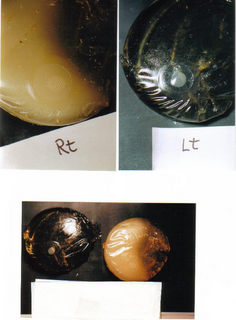
Dr. Pierre Blais holds this Saline Implant after removal
from the body, and he stated that this Breast
Implant
has enough bacteria to kill 20 people ...

Data shows that Saline Implants are badly designed and they leak and or rupture. These faulty products make
an ideal breeding ground for bacteria and mold. See for yourself, the mold and bacteria can be seen in this picture.
Despite spending millions of dollars to portray themselves as defenseless "victims," the breast implant makers
cannot deny that the real victims here are the thousands of women with implants who were deliberately lied to and who are
now suffering debilitating illnesses.

The Facts Are: The manufacturers' own documents reveal a calculated cover-up and campaign of deceit
on the safety of silicone implants.
They have refused to comply with the law requiring them to prove that implants are
safe and effective. Women were told that this product would last a lifetime, but the rupture rate is extraordinarily high.
About 100,000 women have manifested illnesses, with the common link being that they all have silicone breast implants. Two
recent studies could not disprove the link between silicone implants and diseases such as lupus and rheumatoid arthritis.
The studies failed to ask women uniform questions and failed to employ standard laboratory testing.
Manufacturers
have Known for Years that Leaking Silicone Poses Health Hazards
Dow Corning, the leading manufacturer of implants,
ignored doctors' complaints about leaking implants for years. A 1975 Dow Corning memo states that demonstration implants were
"bleeding" and instructed sales staff to wash such implants with soap and water and towel dry before letting doctors handle
them.
A 1977 memo relates how a Dow Corning employee told plastic surgeons "with crossed fingers, that Dow Corning
too had an active contracture/gel migration study underway. This apparently satisfied them for the moment, but one of these
days they will be asking us for the results of our studies." In fact, Dow Corning was not studying contracture, a complication
that occurs when the scar around the implant contracts.
In 1983, Dow Corning's Head of Biomaterial Safety wrote top
company management: "However, I want to emphasize that to my knowledge, we have no valid long-term implant data to substantiate
the safety of gels for long-term implant use." This statement was made 21 years after Dow Corning first put silicone implants
on the market and assured women that implants were safe.
In a 1987 study, the Medtox Project Report, Dow Corning acknowledged
that the chronic reactions to silicone seen in test animals could trigger auto-immune-type diseases in humans.
In
a Bristol-Myers Squibb document from 1985, a company employee states: "Polyurethane has no real history of implantation without
deterioration and we know deterioration products of polyurethane are toxic and in some cases carcinogenic. Whether they are
released in such low levels as to be no threat to the human body -- time will tell."
A 3M document from 1976 states
that "It appears virtually no
documented safety and efficacy data exist on [Don McGhan's] implant products." McGhan's
breast implant company, McGhan Medical Corp., was purchased by 3M.
Manufacturers Refuse to Comply with the Law
Dr. David Kessler, commissioner of the Food and Drug Administration, reiterated in testimony before Congress in August
1995 that "the law requires manufacturers to prove affirmatively, with valid scientific data evaluated by FDA, that their
devices are safe and effective." Why have the manufacturers consistently failed to comply?
Dr. Kessler wrote in the
Journal of the American Medical Association in 1993 that "the adverse effects data on silicone gel implants submitted by the
manufacturers were so poor that the FDA could not determine whether these devices were safe and effective." He added that
the manufacturers' documents suggested that there were inadequate quality control procedures to prevent safety problems and
that problems had been evident for years.
Breast Implants have an Extremely High Rupture Rate, and thus are
Defective
Manufacturers told women that implants would last a lifetime and that ruptures occurred less than 1
percent of the time. But studies published in the American Journal of Radiology in 1992 and the Annals of Plastic Surgery
in 1995 reveal a rupture rate of 5 to 51 percent. A third study, published in Plastic and Reconstructive Surgery in 1993,
ties rupture to the age of the implant. Of implants aged one to nine years, 35.7 percent had ruptured. Of those aged 10 to
17 years, 95.7 percent had ruptured.
FDA Commissioner Kessler wrote in the Journal of the American Medical Association
in 1993 that: "Even with a conservative rupture rate of 5 percent, some 75,000 of the estimated 1 to 2 million women with
implants would be at risk for potentially serious adverse health effects. That is not a safety standard that the FDA can accept."
Thousands of Women Suffering Illnesses Constitute more
than 'Anecdotes'
Dr.
Kessler of FDA states that studies have shown that silicone gel is a potent stimulant to the immune system and could generate
antibodies that attack collagen, a component of connective tissue. In 1992, the FDA received more than 23,000 reports of problems
with implants, including complaints of "bleeding" implants, connective tissue disorders that could lead to arthritis-like
pain and swelling in the joints, fibrous tissue spreading around the implants, and swelling of skin and limbs.
Steven
R. Weiner, associate professor of medicine at the University of California at Los Angeles, asserted in August 1994 that once
you see these women, that's all it takes to be convinced silicone implants can make them sick .... There's no one who has
seen a large number of these women who disagrees."
Safety concerns prompted France in May 1995 to halt the importation,
manufacture, sale or use of silicone-gel breast implants. In May, the Ministry of Health stated that implants filled with
any product other than physiologic saline could rupture and "result in grave danger." France will not allow implants back
on the market "until they have been definitely shown without risk to the user."
Two recent studies are fatally
flawed
One of the most-cited papers used to criticize the link between silicone implants and connective tissue
diseases suffers from fundamental flaws. The "Mayo Clinic" study published in June 1994
(1) failed to ask women uniform
questions or employ standard laboratory testing;
(2) failed to look for the "atypical" signs and symptoms suffered
by women with silicone poisoning;
(3) admitted that the control group (749 women with implants and 1,498 without)
was insufficient and that an accurate study would require 62,000 women with implants and 124,000 without over 10 years;
(4)
suggested an implant failure rate in excess of 30 percent (of the 749 women with implants, 257 had surgical revisions); and
(5) concluded that "No statistically valid conclusions can be drawn from this study."
Further, the study is
at least subject to question since it was
financed in part by the Plastic Surgery Educational Foundation, the educational
arm of the American Society of Plastic and Reconstructive Surgeons. This group has been given hundreds of thousands of dollars
for research by implant manufacturers.
A study published in June 1995 -- the "Harvard" study -- suffers from similar
flaws. Specifically, it
(1) failed to ask women uniform questions or use standard laboratory testing;
(2) failed
to look for signs of "atypical" connective-tissue diseases, the symptoms of which do not fall into any clear diagnostic category;
(3) studied
too few women with breast implants (1,183 women with implants, 876 of which were silicone gel filled);
and (4) failed to account for the fact that women with silicone implants may not manifest illnesses during the first eight
years after implantation.
Further, as with the Mayo Clinic study, author bias is at issue. Two authors of the Harvard
study have admitted under threat of perjury that they are paid consultants to breast implant makers. Also, Dow Corning has
donated $5 million to Boston's Brigham and Women's Hospital, which played a key role in this study. source: www.siegfriedandjensen.co...lants.html
If you or anyone you know has any further questions or problems ... please visit our forum
Implant
Info Net
http://p077.ezboard.com/bimplantinformationnetwork
Frequently Asked Questions
Q:What is the chemical composition of a saline- filled breast implant shell?
A:According to the Office of Device Evaluation (ODE), the office responsible for conducting scientific
reviews of medical devices and radiation emitting products, the following is what is in a saline-filled breast implant shell:
1. 80% high molecular weight silicones
2. 20% finely powdered silica
3. catalyst: small amounts (50-100
parts per million) of a tin or platinum
4. impurities: -small amounts of (1-500 parts per million) of various smaller
silicones -trace amounts of (<5 parts per million) of volatile (readily evaporating) materials like xylene and other organic
compounds (impurities) -trace amounts (<5 parts per million) of metals (impurities)
Q: How do saline implants cause systemic symptoms?
A: The shell surrounding a saline implant is a silicone polymer that can break down due to a lipolysis reaction or
disruption. The silicone from the shell migrates into the capsule surrounding the implants. Cells in the immune system called
macrophages pick up the silicone, break it down into Silica, and then distribute this chemical throughout the body.A chronic
illness may develop that can affect every organ system in the body. Some patients with saline breast
implants have autoimmune,
immune, and neurological disorders similar to patients with silicone breast implants.
Chemical sensitivity also occurs
in patients with saline breast
implants.
Due to immune dysfunction, a patient with saline breast implants may
also have systemic fungal and atypical bacterial infections.
Q: Why did
several doctors tell me that my implants were safe and weren't responsible for my symptoms before I was properly diagnosed
with a silicone-related disease?
A:Many physicians are unaware that silicone or saline implants can cause systemic
symptoms in patients.Silicone-related disease is a controversial topic in medicine.Medical textbooks,medical dictionaries,
etc. do not contain any information about illnesses caused by implants. Also,many physicians think that implants would not
be F.D.A. approved if they were not safe.
Several years ago, you probably wouldn't have found a physician who could
diagnose or provide treatment for illnesses associated with silicone implants.Fortunately, some doctors now understand the
systemic symptoms associated with implants.
Q: How many patients
with implants have a silicone-related disease? The manufacturers of silicone implants state that the percentage of autoimmune
and neurological disorders in implant recipients is the same as the percentage in the general population.
Research
regarding silicone implants is ongoing, but it is now
estimated by reputable sources that 25-30% of all implant recipients
will develop an autoimmune condition.This statistic is much higher than the percentage disclosed by the manufacturers.
Q: What is a "gel bleed"?
A "gel bleed" occurs when the silicone gel in breast implants
slowly leaks through the semi-permeable membrane envelope. The gel then migrates to the capsular area around the implants.
Cells in the immune system called macrophages pick up the gel, break it down into Silica and Silicon, and then distribute
these chemicals throughout the body. The result is immune dysfunction. The silicone gel also causes oxidants to form that
can damage DNA, enzyme systems, and cell walls.
A chronic illness may develop that can affect every organ of the body.
For more information please read Silicone Arthritis and Related
Diseases by Stephen B. Edelson,M.D. et al.
Q: Which types of silicone implants are associated with illnesses?
A:All silicone implants can cause illnesses.This includes
cerebrospinal fluid shunt tubing, slow release hormone
implants, cardiac valves, intraocular lens implants, testicular prostheses, penile implants, digital joint arthroplasty prostheses,
breast implants, pectoral implants, buttock implants, calf implants, malar implants,jaw implants, and chin implants. Saline
implants also can cause systemic symptoms because they have a silicone elastomer shell.
Q: How do implants affect
a patient's career, personal life, and mental abilities?
A:Silicone-related diseases can affect every aspect of
a patient's life. Some patients may be unable to work because of the debilitating symptoms that they have.Interpersonal relationships
may be affected too. Many patients also have cognitive dysfunction and some develop psychological problems; both of these
symptoms may occur in patients
with silicone induced immune dysfunction syndrome.
Some patients with silicone-related
diseases only develop a few symptoms and have a normal life, but others are completely disabled.
Q:I have been diagnosed with a silicone-related disease.When will I begin to feel better after
my implants are removed?
A:Most patients notice an improvement in their health immediately following surgery.Others
improve but do not completely recover. A few remain disabled after explantation.Improvement depends on the age of the patient,
health before implantation, and the duration of the illness. Significant laboratory and clinical improvement should occur
within the first two years after explantation.
Q: Where can
I report an illness or complication that is caused by my implants?
A: Medwatch is the Food and Drug Administration's
program for reporting serious reactions and problems with medical products, such as drugs and medical devices.
Q:Where can I find current information on breast implants?
A:The Center for Devices
and Radiological Health (CDRH) Breast Implant Listserv provides current information on breast implants. The CDRH distributes
a monthly newsletter via email to their subscribers.
http://www.fda.gov/CDRH/
Q:What are the complications associated with chin implants?
A:Complications
associated with chin implants include the following: bone erosion,nerve damage, hematoma, seroma, infection, implant displacement,asymmetry,
mobility, bleeding,swelling,bruising, and silicone-related diseases.
Q:What
are the complications associated with cheek implants?
A:Complications include resorption of the bone under the
implant,nerve damage,blood clot formation, bruising, swelling,bleeding, infection, and silicone-related diseases.
Q: What diagnostic tests are available for silicone?
A:Please refer to the section "Diagnostic
Tests For Silicone-Related Diseases" on this website.
Q: What
illnesses are associated with silicone implants?
A:Clinical syndromes resembling the following disorders are associated
with silicone implants:systemic lupus erythematosus, rheumatoid arthritis, scleroderma, Sjogren's disease, polymyositis, mixed
connective tissue disease, multiple sclerosis ,and other neurological disorders.
Chemical sensitivity is also
associated with implants.
Some patients with immune dysfunction caused by silicone implants have fungal infections(ie
systemic candidiasis) and atypical bacterial infections.
Q: What
are the symptoms of a silicone-related disease?
A:According to Andrew W. Campbell, M.D. , Medical Director of
the Center for Immune, Environmental, and Toxic Disorders,these are the most common symptoms experienced by patients with
silicone prosthetic devices:
breast pain or tenderness
fatigue, usually made worse by exercise
cognitive
function problems, such as attention deficit disorder,
calculation difficulties, memory disturbance, spatial disorientation,
frequently saying the wrong word
psychological problems such as depression, anxiety, personality changes,
mood swings
sleep disturbance and non-restorative sleep
headaches of a greater intensity than before implantation
changes in vision
seizures
loss of balance
numbness and tingling
lightheadedness
paralysis
joint and muscle aches and pains
shortness of breath
lymph node enlargement
weight gain
low
grade fevers
abnormal heart rhythm
hair loss
dry eyes and mouth
frequent canker sores in the
mouth
low back pain
skin changes and/or rashes
severe muscular weakness
intolerance of bright
lights
intolerance of alcohol
decreased libido
ringing in ears
muscle tremors
recurrent
flu-like illnesses
severe allergies
irritable bowel syndrome
night sweats
uncomfortable urination
chest pain
cough
Raynaud's phenomenon
enlarged thyroid.
Q: What should I know
about implants before I have surgery?
A:First,you should consider the reason(s) why you want to have implants.
You should have plastic surgery because you want to enhance your appearance or because you need to have it for reconstructive
purposes.Do not have plastic surgery because someone else (ie your spouse) wants you to do it. Also, there is a condition
called "body dysmorphic disorder" that affects some patients who have plastic
surgery. Patients with body dysmorphic disorder
or B.D.D. have an altered perception of their appearance (ie.they may have an obsession with a minor physical imperfection
which may be imagined)that can affect their ability to have a normal life.You shouldn't have surgery if you think that you
may have B.D.D.
Next, you need to find a plastic surgeon who has experience with the procedure that you want.
Ask your physician how many of his/her patients have had the same procedure. Ask to see photos of other patients who have
had the same procedure performed by that doctor. Ask to speak to other patients who were satisfied with the outcome of their
surgeries.
Finally,you need to understand the complications that may occur with the procedure that you are having.
Ask your plastic surgeon to discuss complications and silicone-related diseases with you before you have surgery.
Q:
I have a silicone-related disease and I feel like my family and friends do not understand what I am going through.I feel "alone".
A: Joining a support network can help you to cope with the medical and personal problems that you are experiencing.
Support groups are located on the internet. There are also chat forums on the internet now where patients can interact with
each for advice and compassion.
You're not alone.
You need to explain to your family and friends what is happening
to you. Maybe they do not understand how serious your condition is. Provide information to them about your disease. Show them
websites and articles that you have regarding silicone-related diseases.
These illnesses not only have an impact
on the patient's life, but also can affect others who are close to them. Perhaps you should encourage your family and friends
to join a support network with you.
Q: If I have a silicone-related
disease and I decide to have children, will they be affected too?
A:It is possible that if you have children,
they might be ill from the toxic effects of silicone. The number of children who are affected by "second generation silicone-related
diseases" is unknown at this time.Smalley et al investigated T cell proliferative responses to silicone dioxide (silica) in
24 childen born to silicone gel breast implant recipients. The authors found that T cells were significantly stimulated by
silicone dioxide. A second part of their study consisted of the investigation of 11 children, four born before implantation,
and seven born after implantation. None of the pre-implant offspring showed T cell responses to silica while five of the seven
post-implant children were positive in this test
|

